Prepubertal Gynecomastia Linked to Lavender and Tea Tree Oils
SUMMARY
Most cases of male prepubertal gynecomastia are classified as idiopathic. We investigated possible causes of gynecomastia in three prepubertal boys who were otherwise healthy and had normal serum concentrations of endogenous steroids. In all three boys, gynecomastia coincided with the topical application of products that contained lavender and tea tree oils. Gynecomastia resolved in each patient shortly after the use of products containing these oils was discontinued. Furthermore, studies in human cell lines indicated that the two oils had estrogenic and antiandrogenic activities. We conclude that repeated topical exposure to lavender and tea tree oils probably caused prepubertal gynecomastia in these boys.
Gynecomastia is generally attributed to conditions that disrupt sex-steroid signaling pathways, resulting in increased or unopposed estrogen action on breast tissue.1 In contrast to gynecomastia in adolescent boys and men, prepubertal gynecomastia is rare and should always be considered pathological, prompting a search for a source of estrogen. Although hyperestrogenemia may be endogenous or exogenous in origin, most persons with prepubertal gynecomastia have normal serum concentrations of sex steroids, and an underlying cause is not identified.2,3 In such cases, possible exposure to exogenous sources of estrogen should be considered. We investigated the cause of prepubertal gynecomastia in three otherwise healthy boys with normal serum concentrations of endogenous steroids.
Case Reports
Patient 1
A boy who was 4 years 5 months old presented with gynecomastia of apparently 2 to 3 weeks' duration. He had no exposure to any known exogenous form of estrogens (ingestants, salves, or ointments). His height and weight were at the 97th percentile and between the 75th and 90th percentiles, respectively. He had bilateral gynecomastia with firm, nontender breast tissue measuring 2 cm by 2 cm in diameter. His testes were 3 ml in volume and of normal consistency. His genitalia were prepubertal (Tanner stage 1). Laboratory investigation showed normal thyroid function; the follicle-stimulating hormone (FSH) concentration was 1.04 IU per liter (reference range, 0.25 to 1.92), luteinizing hormone 0.47 IU per liter (reference range, 0.02 to 1.03), testosterone 0.08 ng per milliliter (0.27 nmol per liter) (reference range, 0.02 to 0.25 ng per milliliter), estradiol less than 20 pg per milliliter (73 pmol per liter) (normal value, <20), dehydroepiandrosterone (DHEA) sulfate less than 5.0 µg per deciliter (0.14 µmol per liter) (reference range, 1 to 40), 17-alpha-hydroxyprogesterone 0.32 µg per liter (0.97 nmol per liter) (reference range, 0.2 to 0.8), and prolactin 8.0 µg per liter per liter (reference range, 2 to 29); the serum biochemistry values, including liver-function tests, were normal. On evaluation 3 months later, the breast buds were tender to palpation and had increased to 2.5 cm by 2.5 cm in diameter with an increased breast mound. The patient's mother reported applying a compounded "healing balm" containing lavender oil to his skin starting shortly before the initial presentation. The gynecomastia partially resolved within 4 months after application of the healing balm was discontinued, at which time the breast buds measured 1.5 cm by 1.5 cm in diameter and were soft in consistency. Several months later, his pediatrician stated that the gynecomastia had resolved completely.
Patient 2
A boy who was 10 years 1 month old presented with a 5-month history of gynecomastia. He and his mother reported that the condition seemed more prominent in the evening and a little less so in the morning. His medical history and family history were unremarkable. His height and weight were above the 97th percentile, and his body-mass index (the weight in kilograms divided by the square of the height in meters) was 21.1. He had firm, tender breast buds, measuring 3.5 cm by 4.0 cm in length and width and approximately 3.5 cm in depth, with stretching of the areolae. His testes were 3 ml in volume and of normal consistency. His pubic hair was Tanner stage 2 (a small amount of long hair at the base of the scrotum), and his genitalia were Tanner stage 1. Laboratory testing showed a testosterone concentration of 0.36 ng per milliliter (1.25 nmol per liter) (normal value, <0.25), free testosterone 0.0066 ng per milliliter (0.0229 nmol per liter) (reference range, 0.0006 to 0.0057), and DHEA sulfate 278 µg per deciliter (7.6 µmol per liter) (normal value, <75). On questioning, it was determined that the patient was not currently using drugs, herbal supplements, or herbal lotions but was applying a styling gel to his hair and scalp every morning and regularly using a shampoo. The labels of both the gel and the shampoo listed Lavandula angustifolia (lavender) oil and Melaleuca alternifolia (tea tree) oil as ingredients. Reevaluation 9 months after use of these products was discontinued showed that his areolar mounds had decreased in depth to approximately 1 cm with almost no palpable glandular tissue.
Patient 3
A boy who was 7 years 10 months old presented with a 1-month history of gynecomastia that had appeared gradually. His height was between the 75th and 90th percentiles, and his weight was at the 50th percentile. He had bilateral gynecomastia with firm, nontender breast tissue that corresponded to Tanner stage 2. His testes were 3 ml in volume and of normal consistency. His genitalia were Tanner stage 1, and there was no pubic hair present. Laboratory testing showed normal thyroid function, FSH 0.49 IU per liter (reference range, 0.25 to 1.92), luteinizing hormone 0.16 IU per liter (reference range, 0.02 to 1.03), estradiol 5 pg per milliliter (18 pmol per liter) (normal value, <10), estriol less than 0.1 ng per milliliter (0.3 nmol per liter) (normal value, <0.1), estrone less than 13 pg per milliliter (48 pmol per liter) (normal value, <13), total estrogens 61 pg per milliliter (225 pmol per liter) (normal value, <130 in adult men), DHEA sulfate 22 µg per deciliter (0.6 µmol per liter) (reference range, 2.5 to 145), 17-alpha-hydroxyprogesterone 0.13 µg per liter (0.39 nmol per liter) (reference range, 15 to 65), and free beta subunit of human chorionic gonadotropin less than 2 mIU per milliliter (normal value, <5); the serum biochemistry values, including liver-function tests, were normal. His history was positive for the use of lavender-scented soap and intermittent use of lavender-scented commercial skin lotions. The gynecomastia resolved completely a few months after use of scented soap and skin lotions was discontinued (personal communication from the patient's family). His fraternal twin used the same skin lotions, but not the lavender-scented soap, and did not have any gynecomastia. Methods Mammalian Cell Culture Human breast-cancer (MCF-7) cells that express estrogen receptors were grown in phenol red-free Dulbecco's modified Eagle's medium containing 10% fetal-calf serum (Atlanta Biologicals), penicillin (100 U per milliliter), and streptomycin (100 µg per milliliter). Human breast-cancer (MDA-kb2) cells that express the androgen receptor were maintained as previously described.4 Cell-culture reagents were obtained from Invitrogen Life Technologies, unless otherwise indicated. For all experiments, the lavender oil (L. officinalis, which is a synonym for L. angustifolia) and tea tree oil (M. alternifolia) (both from Sigma Chemical) were diluted in dimethylsulfoxide before they were added to culture media. Luciferase Assays and Reverse-Transcriptase and Real-Time Polymerase-Chain-Reaction Analysis MCF-7 and MDA-kb2 cells were assayed for luciferase activity with the use of the Dual–Luciferase reporter assay system (Promega) and an LMAX II384 luminometer (Molecular Devices). Total RNA was isolated from MCF-7 cells and MDA-kb2 cells with the use of the RNeasy Mini Kit (Qiagen), according to the manufacturer's protocol. Synthesis of complementary DNA (cDNA) and analyses of gene-specific cDNA concentrations were performed by real-time polymerase chain reaction (PCR), as previously described.5 The PCR primers were designed with the use of Primer Express software, version 2.0 (Applied Biosystems) (see the Supplementary Appendix, available with the full text of this article at www.nejm.org). Statistical Analysis The data were analyzed for statistical significance by the Mann–Whitney nonparametric test.
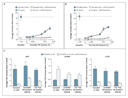
Results Estrogen-Receptor–Dependent Estrogenic Activity In Vitro To determine whether lavender oil and tea tree oil are estrogenic, we performed dose–response experiments in MCF-7 cells that were positive for estrogen receptors and were transiently transfected with an estrogen-inducible luciferase reporter plasmid containing three copies of an estrogen-response element (3X-ERE-TATA-luciferase). Both oils stimulate ERE-dependent luciferase activity in a dose-dependent manner, with the maximum activity observed at 0.025% volume per volume (vol/vol) for each oil, corresponding to approximately 50% of the activity elicited by 1 nM 17β-estradiol (Figure 1A and 1B). Treatment with higher doses of the oils was cytotoxic. The pure estrogen-receptor antagonist fulvestrant inhibited transactivation of the 3X-ERE-TATA-luciferase reporter plasmid by both oils, indicating that their activity is estrogen-receptor–dependent (Figure 1A and 1B). Additional experiments indicated that lavender oil was able to transactivate the estrogen-inducible reporter plasmid in estrogen-receptor–negative SK-BR-3 human breast-cancer cells only after simultaneous transfection with an estrogen-receptor–expression vector (data not shown).
|
Further experiments in MCF-7 cells indicated that the two oils modulated the expression of the estrogen-regulated endogenous genes MYC (also called C-MYC),6 CTSD,7 and IGFBP3.8 Lavender oil and tea tree oil increased the expression of messenger RNA (mRNA) for MYC and CTSD and decreased the expression of mRNA for IGFBP3, as compared with the dimethylsulfoxide controls, in a manner that was similar to the effect of 1 nM 17β-estradiol on the magnitude and timing of the responses (Figure 1C). These responses were attenuated in the presence of 1 µM fulvestrant (Figure 1C).
In Vitro Antiandrogenic Activity
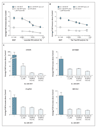
To evaluate the potential androgenic properties of lavender oil and tea tree oil, we performed dose–response experiments in MDA-kb2 cells, a line of human breast-cancer cells that are positive for the androgen receptor and were stably transfected with an androgen-inducible and glucocorticoid-inducible mouse mammary-tumor virus (MMTV)-luciferase reporter plasmid. Treatment of MDA-kb2 cells with the androgen-receptor agonist dihydrotestosterone (DHT) at 0.1 nM, the lowest observed effective dose in this cell line,4 resulted in an increase in luciferase activity that was almost four times higher than that in the dimethylsulfoxide controls (Figure 2A and 2B). In contrast, neither lavender oil nor tea tree oil transactivated the MMTV-luciferase reporter plasmid at any concentration tested (Figure 2A and 2B).
|
The antiandrogenic properties of the two oils were assessed by simultaneously treating the MDA-kb2 cells with DHT and increasing the concentration of lavender oil or tea tree oil. The androgen-receptor antagonist flutamide was also included in these assays, as a positive control for androgen-receptor antagonism. Transactivation of the MMTV-luciferase reporter plasmid by 0.1 nM DHT was inhibited in a concentration-dependent manner by both lavender oil and tea tree oil, as well as by flutamide (Figure 2A and 2B). Maximum inhibition occurred at 0.005% vol/vol for both lavender oil and tea tree oil, corresponding to a decrease in luciferase activity of 52% and 41%, respectively, in the presence of 0.1 nM DHT. The observed inhibitory effects appear to be specific to the androgen receptor, since neither of the two oils attenuated the glucocorticoid-receptor–mediated transactivation of the MMTV-luciferase reporter plasmid in the presence of 5 nM dexamethasone, the lowest observed effective dose in this cell line9 (data not shown). Further experiments in MDA-kb2 cells indicated that the antiandrogenic properties of lavender oil and tea tree oil extended to inhibition of DHT-stimulated expression of the androgen-inducible endogenous genes CYP4F8, C1orf116, UGT2B28, and SEC14L210 (Figure 2C). The antiandrogenic effects of the two oils are not caused by down-regulation of the expression of the androgen receptor, since neither of the oils altered the amount of androgen-receptor mRNA or protein in these experiments (data not shown). Discussion
In contrast to gynecomastia, which occurs in more than 60% of boys during puberty, prepubertal gynecomastia is extremely uncommon. Since there is no known physiologic cause of prepubertal gynecomastia, pathologic causes should be considered. However, a specific cause is rarely identified, and in 90% of patients, prepubertal gynecomastia is labeled idiopathic.2,3 In such patients, the condition may be caused by exposure to an environmental chemical that disrupts the endocrine system and leads to disproportionate estrogen and androgen pathway signaling, a finding reported in a limited number of adults with gynecomastia.11,12
In this report, we describe three otherwise healthy boys with prepubertal gynecomastia, all of whom had normal serum concentrations of endogenous steroids and none of whom had been exposed to any known exogenous endocrine disruptor such as medications, oral contraceptives, marijuana, or soy products.1 The repeated topical application of one or more over-the-counter personal care products that contained lavender oil or lavender oil and tea tree oil was documented for all three patients. Case 1 provided the clinical clue to lavender oil as a potential source, because it was the only topically applied agent used by that child. Use of lavender oil was considered trivial by the child's mother, who acknowledged its use only after repeated questioning. In Case 2, the boy had biochemical evidence of physiologic adrenarche, but the evidence was unrelated to his gynecomastia, which resolved after discontinuation of the use of products containing lavender oil and tea tree oil, despite the persistence of adrenarche. The daily temporal fluctuation in the severity of the gynecomastia reported by the patient's mother might have been caused by the transdermal absorption kinetics of the oils after application each morning. In Case 3, the patient was exposed intermittently to various over-the-counter personal-care products containing lavender oil. His twin brother used the same lotions but not the scented soap, and gynecomastia did not develop in him.
The common use of products containing lavender oil, tea tree oil, or both by the three boys and the resolution of their gynecomastia within months after ceasing use of those products suggest that these oils may possess endocrine-disrupting activity that causes an imbalance in estrogen and androgen pathway signaling. Other components in these products may also possess endocrine-disrupting activity that contributed to the gynecomastia, but those components were not tested because we chose to evaluate only the component that was found in all the products used by the patients (lavender oil) and a chemically similar component that was found in some of the products (tea tree oil).
Our in vitro studies confirm that lavender oil and tea tree oil possess weak estrogenic and antiandrogenic activities that may contribute to an imbalance in estrogen and androgen pathway signaling. Estrogenic or antiandrogenic activities have been reported for other essential oils and some of their monoterpene constituents.13,14,15,16,17,18 On the basis of the three case reports and the in vitro studies, we suspect that repeated topical application of over-the-counter products containing lavender oil or tea tree oil was the cause of gynecomastia in the three patients.
This report raises an issue of concern, since lavender oil and tea tree oil are sold over the counter in their "pure" form and are present in an increasing number of commercial products, including shampoos, hair gels, soaps, and body lotions. Whether the oils elicit similar endocrine-disrupting effects in prepubertal girls, adolescent girls, or women is unknown. Since gynecomastia is labeled idiopathic in approximately 10% of men, one might speculate that unidentified exogenous sources of endocrine-disrupting chemicals may contribute to the onset or progression of the condition, or both, in such patients.1 The results of our in vitro studies indicate a dose–response relationship in the estrogenic and antiandrogenic activities of lavender oil and tea tree oil, suggesting that susceptibility to gynecomastia or other manifestations of endocrine disruption may require exposure to a threshold dose of these oils. The threshold might depend on several undefined factors, including the concentration of the oil in a product; the duration, frequency, and quantity of use of the product; and the genetic characteristics of persons exposed. Until epidemiologic studies are performed to determine the prevalence of gynecomastia associated with exposure to lavender oil and tea tree oil, we suggest that the medical community should be aware of the possibility of endocrine disruption and should caution patients about repeated exposure to any products containing these oils.
Supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (NIEHS).
Dr. Bloch reports receiving grant support and lecture fees from Eli Lilly, Genentech, Novo Nordisk, Pfizer, Tercica, and Serono. No other potential conflict of interest relevant to this article was reported.
We thank the laboratory of Dr. Donald P. McDonnell for the 3X-ERE-TATA-luciferase plasmid; the laboratory of Dr. L.E. Gray, Jr., for the MDA-kb2 cell line; Dr. Grace Kissling (NIEHS) for performing statistical analyses; Drs. Donna Baird, John F. Couse, and Walter J. Rogan for critical review of the manuscript; and Ms. Sue Edelstein for graphical assistance.
Source Information
From the Receptor Biology Section, Laboratory of Reproductive and Developmental Toxicology, National Institute of Environmental Health Sciences, Research Triangle Park, NC (D.V.H., K.S.K.); the Department of Pediatrics, University of Colorado School of Medicine, Denver (N.L., C.A.B.); and Pediatric Endocrine Associates, Greenwood Village, CO (C.A.B.). Address reprint requests to Dr. Korach at the Receptor Biology Section, Laboratory of Reproductive and Developmental Toxicology, National Institute of Environmental Health Sciences, MD B3-02, P.O. Box 12233, Research Triangle Park, NC 27709, or at korach{at}niehs.nih.gov.
References
- Braunstein GD. Gynecomastia. N Engl J Med 1993;328:490-495.
[Free Full Text] - Einav-Bachar R, Phillip M, Aurbach-Klipper Y, Lazar L. Prepubertal gynaecomastia: aetiology, course and outcome. Clin Endocrinol (Oxf) 2004;61:55-60. [CrossRef][Medline]
- Descamps H, Chaussain JL, Job JC. Gynecomastia in boys before puberty. Arch Fr Pediatr 1985;42:87-89. [Web of Science][Medline]
- Wilson VS, Bobseine K, Lambright CR, Gray LE Jr. A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol Sci 2002;66:69-81.
[Free Full Text] - Deroo BJ, Hewitt SC, Peddada SD, Korach KS. Estradiol regulates the thioredoxin antioxidant system in the mouse uterus. Endocrinology 2004;145:5485-5492.
[Free Full Text] - Dubik D, Dembinski TC, Shiu RP. Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cells. Cancer Res 1987;47:6517-6521.
[Free Full Text] - Westley BR, May FE. Oestrogen regulates cathepsin D mRNA levels in oestrogen responsive human breast cancer cells. Nucleic Acids Res 1987;15:3773-3786.
[Free Full Text] - Huynh H, Yang X, Pollak M. Estradiol and antiestrogens regulate a growth inhibitory insulin-like growth factor binding protein 3 autocrine loop in human breast cancer cells. J Biol Chem 1996;271:1016-1021.
[Free Full Text] - Ma R, Cotton B, Lichtensteiger W, Schlumpf M. UV filters with antagonistic action at androgen receptors in the MDA-kb2 cell transcriptional-activation assay. Toxicol Sci 2003;74:43-50.
[Free Full Text] - Doane AS, Danso M, Lal P, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006;25:3994-4008. [CrossRef][Web of Science][Medline]
- Finkelstein JS, McCully WF, MacLaughlin DT, Godine JE, Crowley WF Jr. The mortician's mystery: gynecomastia and reversible hypogonadotropic hypogonadism in an embalmer. N Engl J Med 1988;318:961-965. [Web of Science][Medline]
- Brody SA, Loriaux DL. Epidemic of gynecomastia among Haitian refugees: exposure to an environmental antiandrogen. Endocr Pract 2003;9:370-375. [Medline]
- Perry NS, Houghton PJ, Sampson J, et al. In-vitro activity of S. lavandulaefolia (Spanish sage) relevant to treatment of Alzheimer's disease. J Pharm Pharmacol 2001;53:1347-1356. [CrossRef][Web of Science][Medline]
- Tabanca N, Khan SI, Bedir E, et al. Estrogenic activity of isolated compounds and essential oils of Pimpinella species from Turkey, evaluated using a recombinant yeast screen. Planta Med 2004;70:728-735. [CrossRef][Web of Science][Medline]
- Howes MJ, Houghton PJ, Barlow DJ, Pocock VJ, Milligan SR. Assessment of estrogenic activity in some common essential oil constituents. J Pharm Pharmacol 2002;54:1521-1528. [CrossRef][Web of Science][Medline]
- Dhar SK. Anti-fertility activity and hormonal profile of trans-anethole in rats. Indian J Physiol Pharmacol 1995;39:63-67. [Medline]
- Geldof AA, Engel C, Rao BR. Estrogenic action of commonly used fragrant agent citral induces prostatic hyperplasia. Urol Res 1992;20:139-144. [CrossRef][Web of Science][Medline]
- Chung BH, Lee HY, Lee JS, Young CY. Perillyl alcohol inhibits the expression and function of the androgen receptor in human prostate cancer cells. Cancer Lett 2006;236:222-228. [CrossRef][Web of Science][Medline]